Key Takeaways
- Gene therapy is a broader term used to describe the use of genetic material to treat or prevent disease, usually by introducing new DNA to a cell, whereas gene editing is a specific tool that modifies the existing DNA within a cell.
- The most common gene editing tool currently in use is called CRISPR-Cas9, which is made up of a guide RNA and an enzyme called Cas9.
- Once CRISPR has cut DNA, the cell begins to repair the cut strands of DNA, resulting in “gene editing” and a change in protein production.
Welcome back to the CRISPR Chronicles! In our previous installment, we delved into the fascinating world of gene therapy and how it's revolutionizing the treatment of genetic diseases. Now, we’ll be taking a closer look at a cutting-edge technique called CRISPR-Cas9. This game-changing approach to gene therapy is known as gene editing.
Gene Editing: Precision Possibilities
Gene editing is like a precision tool in the gene therapy toolbox. While traditional gene therapy involves inserting a new gene into cells to replace an existing one, gene editing takes a more targeted approach. Instead of adding a new gene, it focuses on editing the existing DNA within a cell to fix specific mutations that result in too many, not enough, or incorrect proteins being made.
CRISPR-Cas9: The Game Changer in Gene Therapy
Enter CRISPR-Cas9, the rockstar of gene editing. Although there are other mechanisms that can be used to edit genes, CRISPR-Cas9 has garnered attention from the scientific community for its speed, affordability, and reliability. In December 2023, this technology made history as the foundation for the first FDA-approved CRISPR-based therapy, Casgevy, targeting sickle cell disease.
How Does CRISPR-Cas9 Work?
Think of CRISPR-Cas9 as a pair of super precise molecular scissors with a built-in GPS. These scissors are guided by a special molecule called guide RNA, which acts like a map, directing them to a specific spot in our DNA. Once they reach their destination, these molecular scissors, an enzyme called Cas9, snip the DNA at the exact location that needs editing.
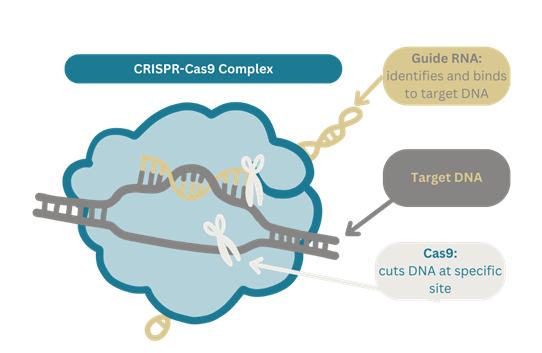
Image credit: Christine Liu for the Innovative Genomics Institute
After the targeted area of DNA has been cut, there are two strands of DNA that are no longer connected, similar to a broken fence. The cell recognizes there's damage that needs repair and deploys a team of proteins to fix the damaged DNA. There are two main approaches a cell uses to do this:
- Non-homologous end joining (NHEJ): This is the most common way the cell repairs the cut DNA. It's like stitching the broken ends of a rope back together, rejoining both strands of DNA. However, sometimes this process introduces small deletions or insertions at the repair site, effectively inactivating or "knocking out" the gene function. This approach is useful for stopping genes from working when they negatively affect cellular function.
- Homology-directed repair (HDR): This is a less common approach where a sequence of DNA is inserted at the site of the cut DNA. Inserting or replacing DNA in this way can fix broken genes or change cellular functions.
CRISPR Considerations: Navigating Challenges in Gene Editing
While CRISPR holds incredible promise, it's not without its challenges and considerations:
- Technical Limitations: Despite its effectiveness, there are important technical considerations and potential risks scientists must address. CRISPR doesn't always cut at the correct site in the genome, and there are limits to which sites it can target. Additionally, predicting or detecting its effects can be challenging.
- Off-Target Effects: One of the main concerns with CRISPR is the potential for off-target effects. These occur when CRISPR makes edits in unintended locations within the genome. These unintended changes could have drastic consequences, such as altering DNA sequences that control cell growth, potentially leading to cancerous cells, or disrupting critical cellular functions.
- Immune System Response: CRISPR proteins can trigger immune system responses in humans, as they originate from bacteria. Strong immune system responses can lead to inflammation and potentially hinder the effectiveness of CRISPR treatments.
- Ethical Considerations: Imagine if we could edit genes in sperm, eggs, or embryos. That could affect not just one person’s health but future generations, too. This type of gene editing is known as “germline gene editing,” and it is a practice that is highly controversial and not currently endorsed or allowed in the United States.
As we continue exploring CRISPR and gene editing, it's crucial to approach these advancements with both excitement and caution. We must carefully consider the potential impact on individuals, families, and future generations that expanding gene editing technology may bring to our doors.
Back to Resources 